simaerep release 0.6.0
Simulate patient-related events in clinical trials with the goal of detecting over and under-reporting sites.
Monitoring reporting rates of patient-related events such as adverse events (AE) in clinical trials is important for patient safety. We use bootstrap-based simulation to assign over- and under-reporting probabilities to each site in a clinical trial. The method is inspired by the ‘infer’ R package and Allen Downey’s blog article: “There is only one test!”.

Key Risk Indicators
Statistical monitoring of clinical trial sites typical employs several key risk indicators which are operational metrics derived from activities at the clinical trial sites. The ratio of AEs is typically one of them, the ratio of issues occurring when treating and examininig patients according to the study protocol might be another one.
Release 0.6.0
Updated Algorithm
The default {simaerep} default algorithm picks for each site a specific evaluation point called visit_med75 based
on the individual progression of the majority of the patients at a given site. Based on this site-level metric patients from the study pool would be sampled. With the most current release v0.6.0 an alternative algorithm which identifies matches on patient-level for bootstrapping has been added. This algorithm has been coded with dbplyr compatible table operations and can be executed within a database-backend. More details about the so called inframe method can be found in the documentation.
Increased Statistical Performance
The latest statistical performance benchmark shows that the inframe method with disabled visit_med75 increases the overall statistical performance of {simaerep}. We can further show that the previously implemented multiplicity correction actually decreases performance of {simaerep}. This observation was already previously made during the validataion of {siamerep} by one of the IMPALA member companies. simaerep() has received a new parameter mult_corr to conveniently disable the multiplicity correction.
Demo
New Recommendation
Recommended Threshold: aerep$dfeval$prob_low_prob_ur: 0.99
suppressPackageStartupMessages(library(simaerep))
suppressPackageStartupMessages(library(tidyverse))
suppressPackageStartupMessages(library(knitr))
set.seed(1)
df_visit <- simaerep::sim_test_data_study(
n_pat = 1000, # number of patients in study
n_sites = 100, # number of sites in study
frac_site_with_ur = 0.05, # fraction of sites under-reporting
ur_rate = 0.4, # rate of under-reporting
ae_per_visit_mean = 0.5 # mean AE per patient visit
)
df_visit$study_id <- "A"
df_visit %>%
select(study_id, site_number, patnum, visit, n_ae) %>%
head(10) %>%
knitr::kable()| study_id | site_number | patnum | visit | n_ae |
|---|---|---|---|---|
| A | S0001 | P000001 | 1 | 0 |
| A | S0001 | P000001 | 2 | 1 |
| A | S0001 | P000001 | 3 | 1 |
| A | S0001 | P000001 | 4 | 2 |
| A | S0001 | P000001 | 5 | 3 |
| A | S0001 | P000001 | 6 | 3 |
| A | S0001 | P000001 | 7 | 3 |
| A | S0001 | P000001 | 8 | 3 |
| A | S0001 | P000001 | 9 | 3 |
| A | S0001 | P000001 | 10 | 3 |
aerep <- simaerep::simaerep(
df_visit,
inframe = TRUE,
visit_med75 = FALSE,
mult_corr = FALSE
)
plot(aerep, study = "A")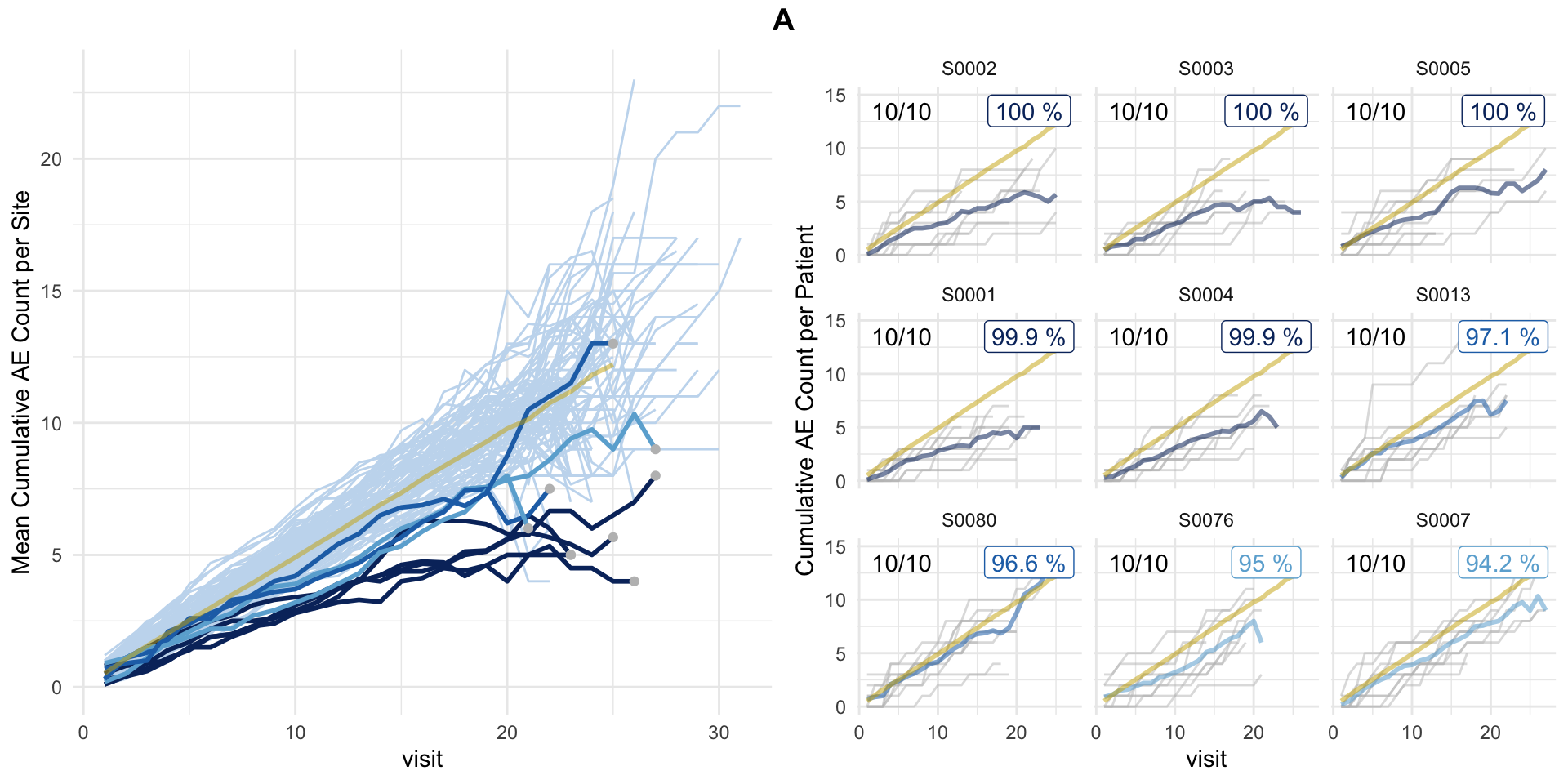
In Database Calculation
We demo in database calculations with duckdb.
We need to provide a in database table that has as many rows as the desired replications in our simulation, instead of providing an integer for the r parameter.
con <- DBI::dbConnect(duckdb::duckdb(), dbdir = ":memory:")
df_r <- tibble(rep = seq(1, 1000))
dplyr::copy_to(con, df_visit, "visit")
dplyr::copy_to(con, df_r, "r")
tbl_visit <- tbl(con, "visit")
tbl_r <- tbl(con, "r")
aerep <- simaerep(
tbl_visit,
r = tbl_r,
inframe = TRUE,
visit_med75 = FALSE,
mult_corr = FALSE
)
plot(aerep, df_visit = tbl_visit)## study = NULL, defaulting to study:A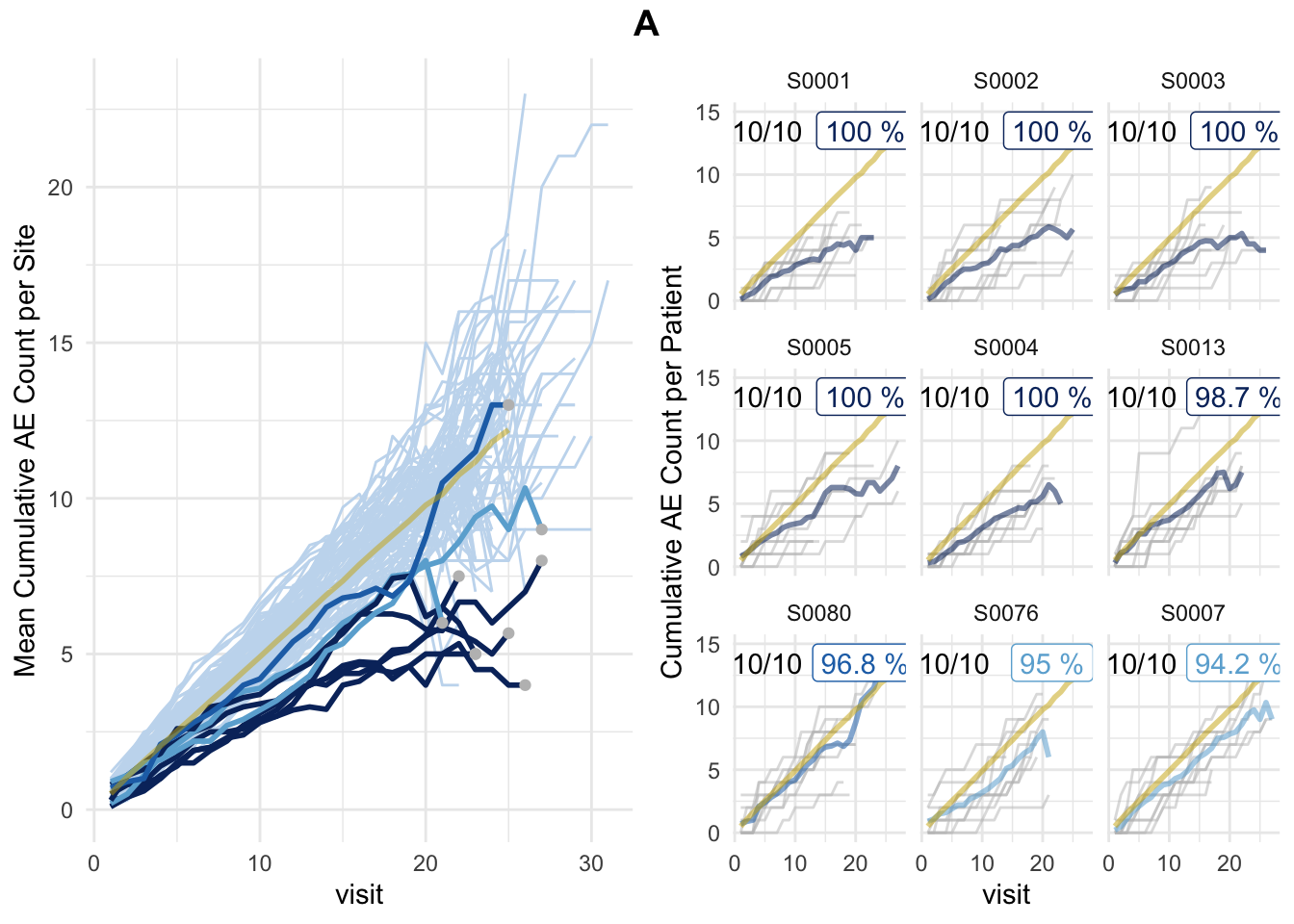
DBI::dbDisconnect(con)Upcoming Breaking Changes
In the upcoming release the default settings of siamerep will be changed to the new recommendations and we will remove the AE wording from the package and change input and output column names.
IMPALA
simaerep has been published as workproduct of the Inter-Company Quality Analytics (IMPALA) consortium. IMPALA aims to engage with Health Authorities inspectors on
defining guiding principles for the use of advanced analytics to complement, enhance and accelerate current QA practices. simaerep has initially been developed at Roche but is currently evaluated by
other companies across the industry to complement their quality assurance activities (see testimonials).
Publications
Koneswarakantha, B., Adyanthaya, R., Emerson, J. et al. An Open-Source R Package for Detection of Adverse Events Under-Reporting in Clinical Trials: Implementation and Validation by the IMPALA (Inter coMPany quALity Analytics) Consortium. Ther Innov Regul Sci (2024). https://doi.org/10.1007/s43441-024-00631-8
Koneswarakantha, B., Barmaz, Y., Ménard, T. et al. Follow-up on the Use of Advanced Analytics for Clinical Quality Assurance: Bootstrap Resampling to Enhance Detection of Adverse Event Under-Reporting. Drug
Saf (2020).
https://doi.org/10.1007/s40264-020-01011-5

